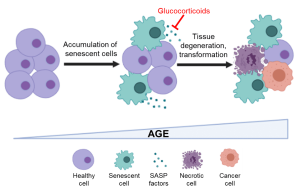
Impact of glucocorticoids on senescence-driven inflammaging. Created with BioRender
Graphic: Aishwarya Iyer-BierhoffPrincipal Investigator: Thorsten Heinzel
Stress induces persistent and functional epigenetic changes, affecting health and lifespan. The hypothalamus-pituitary-adrenal (HPA) axis is the major neuroendocrine circuit that initiates and regulates adaptation to stress, thus promoting survival. Upon encountering stressors, such as an immune challenge, the HPA axis triggers the release of glucocorticoids (GCs) such as cortisol. Cortisol binds to the glucocorticoid receptor (GR) in target cells and activates an anti-inflammatory transcriptional response to mitigate the threat and restore homeostasis. Therefore, low levels of acute stress bolster resilience. During aging, senescent cells that exhibit a pro-inflammatory secretory phenotype (SASP) accumulate and fuel a chronic, systemic, low-grade inflammatory state termed inflammaging. This state accelerates age-related comorbidities such as frailty, neurodegenerative and cardiovascular diseases.
How aging-associated deregulation of the HPA axis and GC-GR signaling impact inflammaging remains unclear. Moreover, GR modulates the chromatin landscape in the vicinity of the DNA methylation sites, linking stress response to epigenetic age. In this study, we will investigate the relationship between epigenetic age, cortisol and pro-inflammatory cytokine levels, and GR expression in aging human cohorts. Using cell culture and animal models, the molecular mechanisms underlying GR-mediated epigenetic regulation of senescence and inflammaging will be interrogated. Moreover, we will address whether anti-inflammatory interventions employing synthetic glucocorticoids can be used as senomodulators to suppress SASP and improve healthspan in model organisms.
Doctoral researcher: Sandra Weber
Postdoctoral researchers: Maren Godmann, Aishwarya Iyer-Bierhoff